We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
- Home
- Sexual Health
Sexual Health
Introducing Verrutop For Sexual Health
Verrutop has been regularly used in a number of European settings for several years and whilst much of the published clinical data spans all aspects of HPV wart therapy from a dermatological perspective there have been a number of standalone reports looking specifically at ano-genital warts which bolster the specific use of Verrutop in this field.
The most recent study, a single blinded RCT post-dates the publication of the EADV Position document.
Verrutop has an impressive and increasing range of clinical studies to support its introduction.
Sign up for more information on Verrutop
Please Click Here to sign up to our mailing list for more information on Verrutop and other products from Espère Healthcare Ltd
Verrutop: included in recent Position Statement
Treatment: Nitric–zinc complex topical solution
Mode of action: Induces a caustic effect on the wart through mummification and protein denaturation/coagulation action
Schedule: Once or up to four times; repeat at 2-week intervals if needed
Clearance rate (%): 90–99
Recurrence rate (%): Not evaluated
Advantages: Efficacy, Easy application
Disadvantages: Current evidence in AGW available from a limited number of patients only, Investigation of recurrence rate is required
Clinical Papers

Treatment of External Condylomas: a retrospective analysis of more than 100 patients treated with an original nitric-zinc and organic acid complex in Belgium and Hungary.
Duray E & Blouard B. Poster. 26th EADV Geneva. 13-17 Sep 2017
- Dual centre retrospective study of 100 patients with external anogenital warts in 2 centres in Belgium and Hungary
- Product applied to each visible condyloma every 2 weeks for a maximum of 4 sessions
- Efficacy defined by 3 level scale measured 2 weeks after last application
Treatment was well tolerated (slight pain, burning sensation during treatment, minimal post-treatment itching, no blistering, no post-treatment pain, and no wound formation.)
results
- Belgian cohort
- 94% of patients had complete cure after a maximum of 3 sessions
- 88.2% were successfully cured after 2 sessions
- 64.7% were successfully cured in just 1 treatment session
- Hungarian cohort
- 91.2% of treated patients were totally cured after a maximum of 4 treatments, once every two weeks
- 84.5 % were successfully cured after 3 sessions
- 77.8 % were successfully cured after 2 sessions
- 48.9% were successfully cured in just 1 treatment session
- 6.7% of patients showed only a partial improvement of their external anogenital warts

A Multicentre, Randomised Clinical Trial to Compare a Topical Nitrizinc Complex Solution Versus Cryotherapy for the Treatment of Anogenital Warts
Pontini P, Mastorino L, Gaspari V, Granger C, Ramoni S, Delmonte S, Evangelista V, Cusini M. 2020
- RCT. Single blinded study Vs Cryotherapy
- 120 patients
- Maximum of 4 treatments
Results
- Complete clearance in 89.7% of the Verrutop group compared with 75.4% in the Cryo group (p=0.0443)
- Recurrences after 1 month 18.4% in Verrutop group vs 38.1% of the cryo group (p=0.0356)
- Recurrences after 3 months 25% of Verrutop group vs 40.6% of cryo group (p=0.149)
Conclusions
Nitrizinc Complex solution can be considered to be as effective as CRYO for the treatment of small (
“We hope that the results of this comparative study will support the inclusion of NZCS as treatment for AGW in future treatment guidelines”

Efficacy Assessment of a Topically Applied Nitric-Zinc Complex Solution for the Treatment of External Anogenital Warts in 100 Patients
Ciccarese G, Drago F, Granger C, Parodi A. 2019
- 100 patients with 418 Ano-Genital warts
- Retrospective Cohort Study
- Verrutop applied every 2 weeks for a maximum of 5 sessions
Efficacy
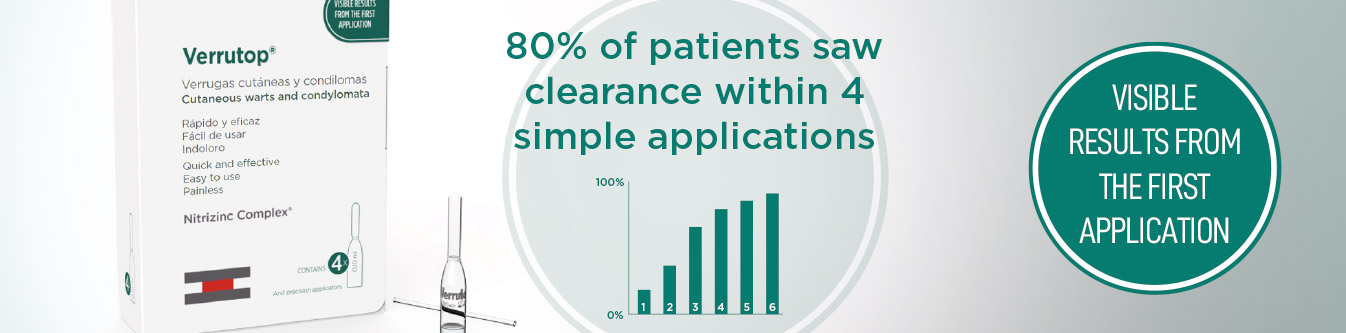
A wart cure rate of 92% was observed in ≤ 4 treatment sessions (383 lesions cured), with a cure rate of 49% with only one application
Complete clearance was observed in 84% of patients
Safety
The treatment was well tolerated by most patients
Follow-up
Relapses were observed in 29% of patients at 3 months and in 5% at 6 months

For more information or to arrange a face to face meeting (or virtual meeting) please call us on 01462 346100 or email chris@esperehealth.co.uk
To Order
Verrutop 4x0.1ml ampoule pack can be ordered directly from Espère Healthcare Ltd by calling us on 01462 346100 and we will deliver within 24-48 hours.
Alternatively, Verrutop is listed under the NHS Supply Chain Sexual Health Framework using NPC code FPH053

Verrutop – changing the management of genital warts – a little drop at a time
Verrutop, the revolution in topical treatment for difficult warts
Verrutop has been regularly used in a number of European settings for several years and whilst much of the published clinical data spans all aspects of HPV wart therapy from a dermatological perspective there have been a number of standalone reports looking specifically at ano-genital warts which bolster the specific use of Verrutop in this field.
We are thrilled to announce that Verrutop has been included in the latest BASHH (British Association for Sexual Health and HIV) Guidelines as a recognised treatment option for ano-genital warts. This inclusion is a milestone in that it recognises Verrutop's effectiveness and safety through three referenced clinical papers, one of which is a direct RCT against cryotherapy. Now, clinics around the country will have added reassurance of the benefits of including Verrutop into their treatment algorithms.



BASHH Recognised
Verrutop is now included in the Guidelines published by the leading UK authority in sexual health care, solidifying its position as a trusted treatment option for ano-genital warts.


Clinically Proven
Backed by robust clinical evidence, the Guidelines have been published in the International Journal of STD & AIDS, Verrutop delivers proven results in the treatment of ano-genital warts.


Simple, Effective Clinic-based Treatment
50% of warts may clear after a single application with no pain, scarring or pigmentation issues.


Cost Effective
Individual patient costs could be as low as £10. Each verrutop box contains 4 ampoules.
RESOURCES
We’re here to help you develop your business, not just supply products to treat your patients. As a small business ourselves we recognise the importance of a little help and support from friends. With this in mind we have created a range of paper and online resources to help bring clients into your clinic, help to educate them and reassure them that you are offering the best possible treatments for their verruca. All these resources are available free of charge with each order and we’ll be updating them regularly.


What is Verrutop?
- Verrutop is formulated with Nitrizinc Complex, a solution containing nitric acid, organic acids, zinc and copper salts.
- It is a topical solution based on Nitrizinc Complex® for treating skin, palmoplantar and periungual warts and condylomata.
- On skin, palmoplantar and periungual warts its effectiveness is 90% with an average of 3 sessions and a maximum of 6. On genital warts, the effectiveness of Verrutop® is over 87% after 3 or 4 sessions, having observed some cases of complete resolution after a single session.


How does it work?
- When Verrutop is applied to a wart, a chemical reaction takes place, which causes mummification of the tissue (dehydration and cellular destruction) and the wart changes colour (white/grey/yellowish).
- Over time, mummification leads to the spontaneous detachment of a wart.


When not to use Verrutop:
- On healthy skin, on inflamed skin or mucous membranes, on malignant cutaneous tumours, on flat warts, on ephelides (freckles) or on keloids (intense scarring reaction).
- On children under the age of 6 years.
- On women who are pregnant or breast feeding.
- On patients with arteriopathy and/or peripheral neuropathies such as seen in some diabetics. In case of a concomitant cutaneous pathology a doctor will decide on the risk/benefit of treatment.
- As concomitant treatment with other topical wart removers.




EFFICACY ASSESSMENT OF A TOPICALLY APPLIED NITRIC-ZINC COMPLEX SOLUTION FOR THE TREATMENT OF EXTERNAL ANOGENITAL WARTS IN 100 PATIENTS
CICCARESE G, DRAGO F, GRANGER C, PARODI A. 2019


A MULTICENTRE, RANDOMISED CLINICAL TRIAL TO COMPARE A TOPICAL NITRIZINC COMPLEX SOLUTION VERSUS CRYOTHERAPY FOR THE TREATMENT OF ANOGENITAL WARTS
PONTINI P, MASTORINO L, GASPARI V, GRANGER C, RAMONI S, DELMONTE S, EVANGELISTA V, CUSINI M. 2020


British association for sexual health and HIV national guideline for the management of anogenital warts in adults (2024)
Nugent, D., Apoola, A., Coleman, H., Gilmour, C., Lawton, M. D., Nori, A., D C Ross, J., Whitlock, G., & Yeend-Curd-Trimble, H. (2024)


TREATMENT OF EXTERNAL CONDYLOMAS: A RETROSPECTIVE ANALYSIS OF MORE THAN 100 PATIENTS TREATED WITH AN ORIGINAL NITRIC-ZINC AND ORGANIC ACID COMPLEX IN BELGIUM AND HUNGARY.
DURAY E & BLOUARD B. POSTER. 26TH EADV GENEVA. 13-17 SEP 2017


Cryotherapy versus topical nitric-zinc complex solution for the treatment of plantar warts: A randomized controlled trial
García-Oreja, S., Álvaro-Afonso, F. J., García-Madrid, M., López-Moral, M., García-Álvarez, Y., & Lázaro-Martínez, J. L. (2023)


Use of Topical Nitric-Zinc Complex Solution to Treat Palmoplantar and Periungual Warts in a Pediatric Population
Giacaman, A., Granger, C., Aladren, S., Bauzá, A., Alomar Torrens, B., Riutort Mercant, M., & Martin-Santiago, A. (2019)


Effect of nitric acid on cutaneous and plantar warts–a case report
Parasuraman, Subramani. (2016). Effect of nitric acid on cutaneous and plantar warts–a case report. Journal of Young Pharmacists. 8. 159-160. 10.5530/jyp.2016.2.21.


Treatment of Erythroplasia and Cervical HPV in a Young Patient with a Coriolus versicolor Vaginal Gel
GÁLVEZ, D. M. G. A. (2023)


A novel Nitrizinc Complex® solution for topical treatment of seborrhoeic keratosis: an interventional clinical study
Tribó, María José et al. European journal of dermatology : EJD vol. 29,2 (2019): 203-208. doi:10.1684/ejd.2019.3525


For more information or to arrange a face to face meeting (or virtual meeting) please call us on 01462 346100 or email chris@esperehealth.co.uk


Verrutop is listed under the NHS Supply Chain Sexual Health Framework using NPC code FPH053


Verrutop 4x0.1ml ampoule pack can be ordered directly from Espère Healthcare Ltd by calling us and we will deliver within 24-48 hours.